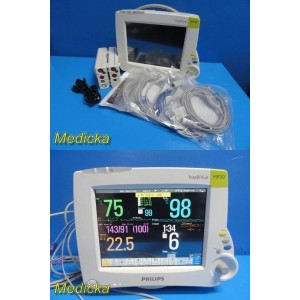
Philips IntelliVue MP30 Mon...
Philips IntelliVue MP30 Monitor W/ MMS+Hemodynamic Module, Batteries,Leads~38805
Item specifics
- Used
- Model
- M8002A
MPN- 862135
Product details
Manufacturer | Philips Healthcare |
Item: | Philips IntelliVue MP30 Monitor W/ MMS+Hemodynamic Module, Printer, Batteries & Leads |
Model/Cat # | MP30 / M8002A |
Serial No. | DE72867133 |
Made In: | Germany |
Date of Manufacture: | 07-2009 |
Voltage: 100–240V~, 50/60Hz, 1.8–1.0A
Short description:
Philips IntelliVue MP30 patient monitor configured with MMS module, hemodynamic extension module, integrated printer, and dual Philips M4605A lithium-ion batteries installed. The monitor has been powered on, tested, and found to be working well with a bright, responsive touchscreen interface.
Software Version: G.01.73
Installed Options: H10, A04, C01, C02, C05, C15, C20
Profiles: Adult, Pediatric, Pediatric SpO₂ / NIBP, Baby
Screen Options / Display Modes:
- Three-Wave / One-ECG Screen NBP SpO₂ Screen Four-Wave Pressure Screen
- Four-Wave ECG + NBP Trend Screen Big Numerics Horizon Screen Transducer Screen Multi-Lead ECG Arrhythmia / ECG Screens
- PANZ Screen
Condition:
- LCD: Bright and clear with one light cosmetic scratch only
- Touchscreen: Fully responsive to all touch commands
- Front Bezel & Rear Housing: Intact; no dents or cracks
- Cosmetics: Minor scratches and scuffs consistent with prior clinical use
- Handle: Firm and intact
- Overall Condition: Very good cosmetic and operational condition
Functionality Summary:
- Device powers on normally and boots without error
- Touchscreen navigation is smooth and accurate
- All installed modules are recognized by the system
- Printer is installed and recognized (paper not included)
System-wide measurements supported:
ECG / RESP
SpO₂ (Nellcor type)
NIBP
Temperature
Pressure (IBP / CVP / ART capable)
All parameters tested except IBP, which could not be pressure-verified due to lack of transducers (disposable items)
BATTERY CONFIGURATION:
Battery Slots: Dual
Installed Batteries: 2 × Philips M4605A Lithium-Ion Batteries
10.8V | 65Wh
- Batteries hold charge after overnight charging
- Original battery life remaining is unknown (used batteries)
MODULE CONFIGURATION:
1. MMS Module:
- Model: M3001A
Reference Number: 862442 - Option: A03C06
- Serial Number: DE9070BDEG
- Functions: ECG/RESP, SpO₂, NIBP, Pressure, Temperature
- Condition: Very good; connectors intact; light cosmetic wear only
2. Hemodynamic Extension Module:
- Model: M3012A
- Reference Number: 886-2111
- Serial Number: DE83731537
- Date of Manufacture: 11 / 2009
- CE: 0366
- Condition: Fully seated and recognized; module release latches intact
- Note: Small cosmetic stress crack on superior surface (see images); does not affect function
3. Recorder / Printer:
- Integrated right-side printer module
- Printer recognized by system
- Recorder paper not included
WHAT IS INCLUDED:
Equipment
- Philips IntelliVue MP30 Monitor
- MMS Module
- Hemodynamic Extension Module
- Integrated Recorder / Printer
- 2 × Philips M4605A Batteries (installed)
- Power Cord
New Accessories (Non-OEM unless specified)
- ST1305 Reusable Temperature Probe
- SG5124S ECG Trunk Cable + 5-Lead Set
- SP9325A Adult Direct-Connect SpO₂ Sensor (Nellcor type)
- SH0910S NIBP Hose
- SC2711-11 Adult Reusable NIBP Cuff
- SB0411 IBP Adapter Cable (Medex / Abbott style)
WHAT IS NOT INCLUDED:
- CO₂ module
- Additional hemodynamic or specialty modules beyond those listed
- Recorder paper
- Rolling stand or bedrail mounts
- User manual
BUYER NOTES:
- IBP functionality not pressure-tested due to lack of transducers
- Batteries are used and included as-is
- Device is pre-owned and not patient-ready
- Calibration, certification, and clinical validation are the responsibility of the buyer
- Please review all uploaded images carefully for cosmetic details
*Items shown in photos are the items that will ship. If it is not shown in the photos, it is not included*
Stock ID : 38805 {P.O 5007} (R2 C6)
Comments:
Kindly enlarge the images and see them with zooming.
Concern or query: Ask a Question
Condition: USED, please refer to images for cosmetic condition.
- 1. Overall Condition: As seen in images - Very Good
- 2. Physical Condition: Please review images before bidding - Very Good
- 3. Working Condition: Has been tested at our end & functionality has been shown in the images......Will receive item as pictured…………(Offered AS IS)
- 4. “As a whole; the item has not been tested technically or professionally at our end- unable to test --- being sold STRICTLY AS IS! Biomed check is strongly recommended before clinical use!”
- 5. Completeness: (with everything seen in the pictures. Please look at all pictures and go through detail description before bidding.)