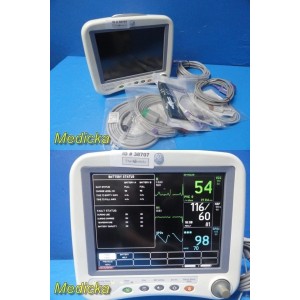
2013 GE Dash 4000 Monitor W...
2013 GE Dash 4000 Monitor W/ ECG, NIBP, SpO2, Temp Leads & 2x Batteries ~ 38707
Item specifics
- Used
- Model
- Dash 4000
MPN- 2035598-203
Product details
Manufacturer | GE Medical Systems |
Item: | 2013 GE Dash 4000 Monitor W/ ECG, NIBP, SpO2, Temp Leads & 2x Batteries |
Model/Cat # | Dash 4000 / Ref 2035598-203 |
Serial No. | SHQ13454418SA |
Made In: | USA |
Date of Manufacture: | 2013-11 |
Voltage: 100-240V 50-60Hz 2.0-1.0A
Short description:
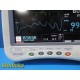
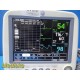
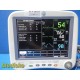
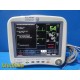
The GE Dash 4000 is a high-acuity, transport-ready patient monitor designed for ICU, OR, ED, and step-down environments. This unit powers on normally, passes internal diagnostics, and all installed parameters—ECG, NIBP, SpO₂, Respiration, and Temperature (TP1)—are active and responsive. The printer module is not installed. Two GE lithium-ion batteries are installed and fully charged at the time of testing. Buyer is advised to charge the batteries overnight before clinical use; no warranty is provided on used batteries.
CE Mark: CE 0459
NBP Technology: DINAMAP® SuperSTAT
SpO₂ Technology: Nellcor OxiMax® compatible
Software Configuration:
- Admit Menu Type: Standard
- Monitor Type: Adult – ICU
- Enabled Software Options: 0 BP option displayed (no IBP board installed), Network, Cardiac, Atrial Fibrillation, ECG IntelliRate
- CO₂ Hardware: Not Installed
- ECG, NIBP, SpO₂, Respiration (RR), Temperature TP1, Alarms
Overall Condition: Excellent, fully functional for installed parameters
- Screen: Bright, clear, no dead pixels
- Casing: Clean, no cracks, dents, or stress marks
- Surface: Light scuffs from normal prior use
- Handle: Secure and intact
- Printer: Not installed
- Batteries: Two installed GE Li-ion packs, both fully charged during testing
Performance:
Unit boots normally and completes internal self-tests
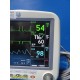
ECG waveform stable
NIBP cycles and measures correctly
SpO₂ responds appropriately with included sensor and adapter cable
Respiration and TP1 temperature channels active
Alarms present and adjustable
Cosmetic Rating: 9/10
- Battery A: Installed – fully charged at testing
- Battery B: Installed – fully charged at testing
- (1) GE Dash 4000 Patient Monitor (REF 2035598-203)
- (1) New NIBP Hose (REF SH3931D)
- (1) New Adult NIBP Cuff 25–35 cm (REF SC1721-3232)
- (1) New ECG Cable, 5-Lead SNAP AHA (REF SG5112S)
- (1) Reusable SpO₂ Sensor (REF SP91198) – NEW
- (1) Reusable SpO₂ Adapter Cable DOC-10 (REF 2021406-001) – USED
- (1) New Reusable Temperature Probe, Skin Type (REF ST1304)
- (1) USA Power Cord
- (1) Device Stand Base Plate
- (2) GE Original Rechargeable Batteries – installed
- CO₂ module or sampling accessories
- IBP hardware, cables, or transducers
- Printer module
- Additional accessories or disposables
- Printed user manual
*Items shown in photos are the items that will ship. If it is not shown in the photos, it is not included*
Stock ID : 38707 (P.O 4018) (R2 C1)
Comments:
Kindly enlarge the images and see them with zooming.
Concern or query: Ask a Question
Condition: USED, please refer to images for cosmetic condition.
- 1. Overall Condition:- As seen in images - Very Good
- 2. Physical Condition: Please review images before bidding - Very Good
- 3. Working Condition: Has been tested at our end and the functionality has been shown in the image section......Will receive item as pictured ……………(offered AS IS)
- 4. “As a whole; the item has not been tested technically or professionally at our end- unable to test --- being sold STRICTLY AS IS! Biomed check is strongly recommended before clinical use!”
- 5. Completeness: (with everything seen in the pictures. Please look at all pictures and go through detail description before bidding.)