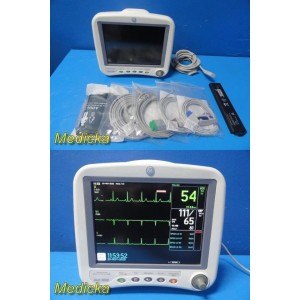
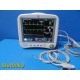
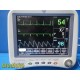
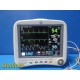
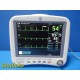
GE Dash 4000 Monitor (Masim...
GE Dash 4000 Monitor (Masimo SpO2, NIBP SuperSTAT, ECG, TEMP/CO) & Leads ~38709
Item specifics
- Used
- Model
- Dash 4000
MPN- 2035598-202
Product details
Manufacturer | GE Medical Systems |
Item: | GE Dash 4000 Patient Monitor (2x IBP, SpO2, NIBP, ECG, Temp) W/ NEW Leads |
Model/Cat # | Dash 4000 / Ref 2035598-202 |
Serial No. | SHQ13444167SA |
Made In: | USA |
Date of Manufacture: | 2013-10 |
Voltage: 100–240 V~ (50–60 Hz, 2.0–1.0 A)
Short Description:
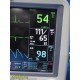
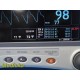
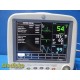
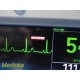
The GE Dash 4000 is a compact, durable, high-acuity patient monitor designed for ICU, ER, OR, and transport use. This unit has been powered on, tested, and all installed parameters are working (ECG, SpO₂, NIBP, Temperature, RR). The display is bright, the trim knob is responsive, and the printer mechanism is functional. Although this unit has hardware for 2× IBP, the software license is not activated, so IBP will not appear as a selectable parameter.
CE Mark: CE 0459
NBP Technology: DINAMAP® SuperSTAT
SpO₂ Technology: Masimo SET®
Software Configuration:
- Admit Menu Type: Standard
- Patient Monitor Type: Adult – ICU
Enabled Software Options:
• NIBP
• ECG IntellIRate
• (No IBP enabled despite installed hardware)
• No advanced options installed (CO₂, Cardiac Output, etc.)
Installed Hardware (Detected on side Ports):
2 × IBP ports (hardware present)
Important: IBP not enabled in software → IBP will not function unless licensed.
Installed & Working Parameters (Verified)
- ECG: ON
- NIBP: ON
- SpO₂ (Masimo SET®): ON
- Temperature (TP1): ON
- Respiration (RR): ON
- Alarms: ON
- Printer: Installed and operational (device shows NO PAPER message only when empty → mechanism working)
Battery Status:
- Battery A: Not installed
- Battery B: Installed, but “NO COMM” → likely dead or non-communicicative
➤ Replace both batteries for portable operation.
➤ Unit operates perfectly on AC power.
Condition:
- Overall Condition: Very Good Used
- Cosmetic Rating: 8/10
- LCD: Bright, clear, no cracks
Housing:
• Small chip on upper right corner
• Minor, inconspicuous stress cracks on back bezel
• Typical handling scuffs- Handle: Intact
- Feet: All present and stable
- Buttons/Trim Knob: Fully responsive
- Ports & Connectors: All tested and functional
- Printer: Installed and mechanically functional
Functionality Summary:
- Passed power-on self-test
- All installed parameters verified with accessories
- Waveforms stable
- NIBP functional with DINAMAP® SuperSTAT
- Masimo SET® SpO₂ responsive
- Temperature probe tested
- Printer mechanism works
- Keys, menus, and softkeys responsive
- IBP hardware detected but software license not installed
Included:
- (1) GE Dash 4000 Patient Monitor – REF 2035598-202
- (1) Masimo SET® Direct SpO₂ Sensor (New) – REF SP9310W
- (1) ECG 5-Lead AHA SNAP Cable (New) – REF SG5112S
- (1) New NIBP Hose – REF SH3931D
- (1) Adult NIBP Cuff 25–35 cm (New) – REF SC1721-3232
- (1) Reusable Temperature Probe (Skin) – REF ST1304
- (1) Power Cord (USA)
Batteries:
- Battery A: Not installed
- Battery B: Installed but non-communicative (dead)
Not Included:
- IBP Transducers / Cables
- CO₂ Module
- SpO₂ Extension Cable (not required for direct sensor)
- Mounts, stands, or bed brackets
- Paper for printer
- User manual (hard copy)
Comments:
This Dash 4000 shows very good cosmetics, a bright display, and fully functional monitoring of ECG, SpO₂, NIBP, RR, and Temperature.
The device contains IBP hardware, but IBP is not licensed in software, so it is not available as a parameter.
Batteries are non-functional or missing, so external power is required unless replacements are installed.
Ideal for facilities requiring a reliable bedside or transport monitor operating on standard ICU parameters.
Shipping Note:
- Weight (Packed): ~20 lbs
- Dimensions: 18″ × 18″ × 16″
- Packaging: Double-wall box, antistatic wrap, foam inserts
- Ships Within: 1–2 business days
- Carrier: FedEx Ground / UPS Ground
*Items shown in photos are the items that will ship. If it is not shown in the photos, it is not included*
Stock ID : 38709 (P.O 4018) (R2 C2)
Comments:
Kindly enlarge the images and see them with zooming.
Concern or query: Ask a Question
Condition: USED, please refer to images for cosmetic condition.
- 1. Overall Condition: As seen in images - Very Good
- 2. Physical Condition: Please review images before bidding - Good
- 3. Working Condition: Has been tested at our end and the functionality has been shown in the image section....Will receive item as pictured…………(Offered AS IS)
- 4. “As a whole; the item has not been tested technically or professionally at our end- unable to test --- being sold STRICTLY AS IS! Biomed check is strongly recommended before clinical use!”
- 5. Completeness: (with everything seen in the pictures. Please look at all pictures and go through detail description before bidding.)