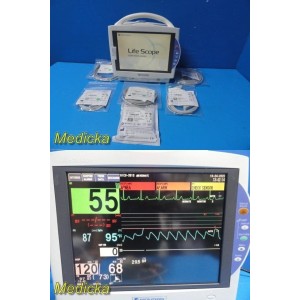
Nihon Kohden BSM-6501A Beds...
Nihon Kohden BSM-6501A Bedside Monitor W/ AY-653P Module & NEW Leads ~ 38201
Item specifics
- Used
- Model
- BSM-6501A
MPN- MU651RA
Product details
Device Information:
- Manufacturer: Nihon Kohden Corporation
- Model / Series: Life Scope BSM-6000 Series
- System Reference Number: BSM-6501A
- Main Unit Reference Number: MU-651RA
- Serial Number: 02879
- Date of Manufacture: 2014
- Parameter Module Reference Number: AY-653P
- Module Serial Number: 03221
- Module Date of Manufacture: 2011
- Module Software Version: 03–10
- Voltage Requirement: 100–240V AC, 50/60Hz, 90VA
- Country of Origin: Tokyo, Japan
- Certifications: CE Marked (0086), BIS Ready
- Safety Classification: Not MR Safe
- Installed Parameters on Module: 3 × IBP, 2 × TEMP, SpO₂ (Masimo SET technology), NIBP, ECG/RESP, ECG/BP Out
Short Description:
This listing features a Nihon Kohden Life Scope BSM-6501A Multi-Parameter Patient Monitor complete with the AY-653P parameter module. This BIS-ready system supports advanced Masimo SET SpO₂ monitoring and multiple physiological parameters, offering reliable patient monitoring for clinical and hospital environments. The system includes a full-color touchscreen display, bright and responsive LCD, and a modular design for versatile use.
Condition:
- Overall Condition: Excellent, pre-owned and tested.
- Cosmetic Condition: No dents or cracks; light cosmetic wear such as minor scratches or scuff marks from prior handling.
- Screen Condition: Bright, scratch-free LCD display, fully touch-responsive, and navigation confirmed.
- Mechanical Condition: Top module latch and all terminal connectors are intact and secure. Handle is firm and undamaged.
- Battery Compartments: Two slots available; no batteries included.
- Printer: Not installed.
- Mounting Plate: Included and securely attached to the unit.
- Regulatory Note: Device is not MR safe but is BIS monitoring ready.
Functionality Summary:
The unit powers on successfully, passes self-tests, and all control panel and touchscreen functions respond correctly.
- Parameters verified through system setup: ECG, RESP, NIBP, TEMP, SpO₂ (Masimo), IBP, and ECG/BP out.
- Displayed test readings confirm proper function of installed software (Version 05–21).
- Internal navigation and alarm menus are responsive, and system software is stable.
- No hardware errors noted during extended runtime testing.
What’s Included:
- Nihon Kohden BSM-6501A Monitor
- AY-653P Multi-Parameter Module
New Sino-K compatible accessories (non-OEM):
ECG Cable (REF SG5123, 5-lead)
Reusable SpO₂ Sensor (REF SP9321E, Masimo type, adult)
Reusable NIBP Cuff (REF SG7111-3232, adult 25–35cm)
NIBP Adapter Hose (REF SHT2131D)
Reusable Temperature Probe (REF ST307)
Hospital-grade Power Cord
What’s Not Included:
- Batteries
- Built-in Printer
- Rolling cart, wall mount, or pole clamp
- User manual, consumables, or disposable accessories
*Items shown in photos are the items that will ship. If it is not shown in the photos, it is not included*
Stock ID : 38201 (P.O 4017) (WH104 SCC13)
Comments:
Kindly enlarge the images and see them with zooming.
Concern or query: Ask a Question
Condition: USED, please refer to images for cosmetic condition.
- 1. Overall Condition: As seen in images : Very Good
- 2. Physical Condition: Please review images before bidding : Very Good
- 3. Working Condition: Has been tested at our end and the functionality has been shown in the image section....Will receive item as pictured…………(Offered AS IS)
- 4. “As a whole; the item has not been tested technically or professionally at our end- unable to test --- being sold STRICTLY AS IS! Biomed check is strongly recommended before clinical use!”
- 5. Completeness: (with everything seen in the pictures. Please look at all pictures and go through detail description before bidding.)